This blog was originally published as an opinion piece in STAT here.
In the midst of a deadly epidemic like Ebola, who should get an experimental vaccine that provides protection against the disease 97.5% of the time?
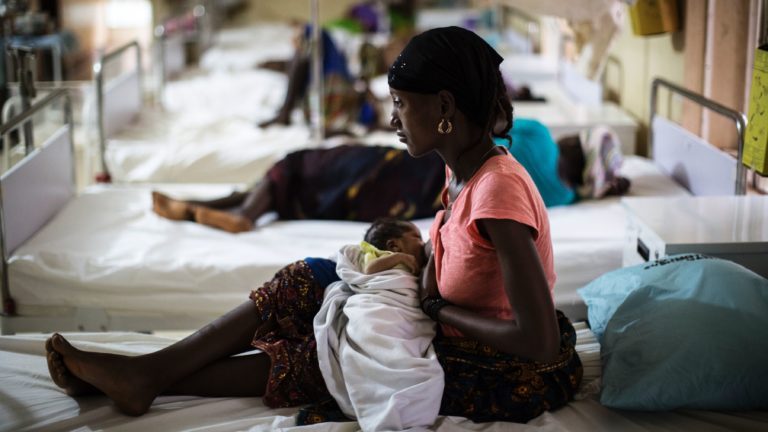
The obvious candidates would be immediate contacts of people who developed Ebola, and their contacts. Yet an entire group of vulnerable individuals who fall into these categories — women who are pregnant or who are breastfeeding infants — are being denied this lifesaving intervention.
The vaccine, called rVSV-ZEBOV, which is being developed by Merck, has not yet been approved for commercial use. The vaccine is currently being used in the Democratic Republic of the Congo, where more than 2,000 people have been infected with the Ebola virus and close to 1,400 have died, making this the world’s second-largest Ebola outbreak.
So far, more than 130,000 people in the DRC outbreak have received rVSV-ZEBOV using what’s called a ring vaccination approach: The vaccine is offered to contacts of people diagnosed with Ebola, their contacts, and front-line workers — unless they are pregnant or lactating. More than 300 pregnant women and more than 600 breastfeeding women identified as contacts since the vaccination campaign began have been denied the opportunity to get rVSV-ZEBOV.
One rationale for withholding the vaccine from women who are pregnant or lactating is that the vaccine had not been tested in this group to evaluate its effects on the fetus or breastfeeding children.
In February, the DRC announced that women who are pregnant and lactating, as well as children under the age of 1, should be offered access to rVSV-ZEBOV, reversing a previous decision that had drawn fire from public health experts.
Last week, an ethics committee of the University of Kinshasa’s School of Public Health endorsed this strategy by approving an amendment to the vaccination protocol, allowing vaccination of pregnant contacts who are beyond the first trimester of pregnancy and those who are lactating.
This is an encouraging step forward, and one we hope will quickly translate into ensuring that pregnant and lactating women who can benefit from the vaccine will get it.
But it doesn’t go far enough. We believe that the criteria for offering the vaccine should apply regardless of pregnancy or lactation status: The vaccine should be offered not only to pregnant and lactating women who are contacts of those diagnosed with Ebola, but also to pregnant and lactating health care workers, and to pregnant and lactating women who are the contacts of contacts. To guide future vaccination efforts, data should be collected on pregnancy outcomes. We also believe that pregnant women should be offered the vaccine regardless of trimester.
There are compelling reasons why it is critical that pregnant women not be excluded from current Ebola vaccination strategies. Not only do pregnant women become infected with Ebola, but women appear to have higher rates of infection than men, either because of their traditional role as caregivers for ill family members or because of an increased susceptibility to the virus.
The risk for death is at least as high among Ebola-infected pregnant women as among women who are not pregnant, and may be higher. In addition to the risk posed to the pregnant woman herself, it is also important to consider the benefits and risks of vaccination for the fetus and newborn, since Ebola infection during pregnancy presents serious threats to their health: Pregnant women infected with Ebola are at very high risk of miscarriage, stillbirth, or neonatal death.
Given the risk of Ebola to pregnant women and their babies, the benefits of their receiving the vaccine to prevent Ebola illness and death are clear.
To be sure, weighing the potential risks of the vaccine on a pregnant woman and her fetus is complicated. No data are available on the effects of this experimental Ebola vaccine on pregnant women or on their fetuses. There are, however, many years of experience with other vaccines in pregnancy that may help frame this issue.
For many years, pregnant women in developing countries have been given the tetanus toxoid vaccine to prevent babies from dying of tetanus, and there is no evidence of harmful effects on the fetus. Inactivated influenza vaccine along with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (known collectively as Tdap) are routinely recommended during pregnancy. Not only have these vaccines been shown to be safe, but they have an added benefit: Antibodies produced by the mother cross the placenta to the fetus, providing the newborn with protection against influenza and pertussis, both deadly illnesses in infants, for the first few months of life.
The experimental Ebola vaccine is a live attenuated vaccine, and live vaccines have historically not been given during pregnancy because of the theoretical risk that the weakened virus might cross the placenta and infect the fetus. Yet when pregnant women have inadvertently been vaccinated with live vaccines, no harmful effects have been observed. And recommendations for one live vaccine — the yellow fever vaccine — emphasize weighing the risks and benefits for pregnant women, given the severity of illness potentially prevented by the vaccine.
Lessons from dolutegravir, a treatment for HIV
A recent experience with a new HIV medication called dolutegravir offers some guidance here. Dolutegravir has been recognized as having many benefits for treatment of HIV, given its ability to clear the virus quickly and consistently with fewer side effects and lower cost. Yet its use was limited among pregnant women due to concerns about fetal safety.
In July of 2018, interim data from a study in Botswana suggested that women who take dolutegravir early in pregnancy might be at increased risk of having a baby with a neural tube defect, such as spina bifida or anencephaly. While waiting for more data, recommendations were made that women who are planning a pregnancy or who aren’t using consistent contraception should not take dolutegravir.
Since then, modeling data have suggested that even if dolutegravir does cause neural tube defects, women are less likely to die and their children would be more likely to be alive and HIV-free if given this drug because its benefits outweigh the risks. Perhaps more important, following a stakeholder meeting of African women living with HIV to discuss the safety signal, the group issued this powerful consensus statement: “It is critical to not just view a pregnant mother, or any women of childbearing potential, as a vessel for a baby, but as an individual in her own right, who deserves access to the very best, evidence-based treatment available and the right to be adequately informed to make a choice that she feels is best for her.”
Subsequent guidance for the use of dolutegravir has recognized this issue: The WHO currently recommends that women should be counseled regarding the potential benefits and risks of dolutegravir and allowed to choose to use it or an alternative drug.
For women at high risk for contracting Ebola, rVSV-ZEBOV represents an even more stark example of a potentially lifesaving intervention. Ebola kills women and their babies. There is an ethical obligation to not exclude women from a protective intervention like rVSV-ZEBOV because they are pregnant or lactating. They should be given the best available information to make a decision about being vaccinated and, if they choose to go forward, should be offered the Ebola vaccine regardless of pregnancy or lactation status.
Since the identification of thalidomide as a cause of limb defects in the 1960s, clinicians and researchers have emphasized protecting the fetus from harmful exposures. While this is a laudable goal, pregnancy shouldn’t automatically exclude women from receiving lifesaving therapies. Pregnant women and their fetuses deserve the opportunity to be protected from severe disease and death. As with the general population, the focus needs to remain on the benefits of the intervention and whether those outweigh the potential risks.
Based on what is known about the severe effects of Ebola virus on a woman and her fetus and preliminary data on the effectiveness of the Ebola vaccine, the benefits of Ebola vaccine outweigh the potential risks, even during the first trimester when the fetus’s organs are forming.
The way forward should be clear: The Ebola vaccine should be offered to lactating and pregnant women regardless of pregnancy trimester to protect women and their fetuses from severe illness and death.